Water Phase Change Visualization
Exploring latent heat and phase transitions in water at 1 atm pressure
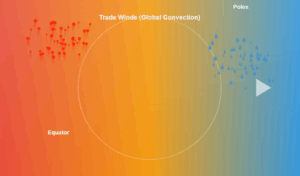
Temperature vs Heat Energy
Molecular Simulation
Understanding Latent Heat
What is Latent Heat?
Latent heat is the energy absorbed or released by a substance during a phase change without a change in temperature.
Where:
- Q = Heat energy (Joules)
- m = Mass of the substance (kg)
- L = Latent heat (J/kg)
Phase Changes in Water
Water undergoes phase changes at specific temperatures under 1 atm pressure:
- Melting Point: 0°C (solid → liquid)
- Boiling Point: 100°C (liquid → gas)
During these phase changes, temperature remains constant while energy is absorbed or released.
Latent Heat of Fusion
(Melting ice at 0°C)
Latent Heat of Vaporization
(Boiling water at 100°C)
Why Steam Burns Are More Severe
Steam at 100°C carries 22.6 × 10⁵ J/kg more heat than water at 100°C. When steam condenses on your skin, it releases this large amount of latent heat in addition to the heat from cooling, causing more severe burns than boiling water.
This demonstrates the significant amount of energy stored in water vapor during the phase change from liquid to gas.