Metallic vs Non-metallic Properties
Interactive comparison tool for physical and chemical properties
METALLIC
NON-METALLIC
Sodium (Na)
11
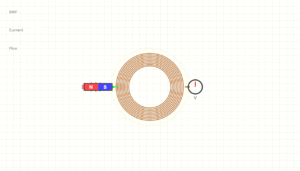
Electrical Conductivity:
21.0 MS/m
Melting Point:
97.8°C
Density:
0.97 g/cm³
Electronegativity:
0.93
Ion Formation:
Na⁺
Electron Behavior:
Donates 1 electron
Chlorine (Cl)
17
Electrical Conductivity:
0.00001 MS/m
Melting Point:
-101.5°C
Density:
0.003 g/cm³
Electronegativity:
3.16
Ion Formation:
Cl⁻
Electron Behavior:
Accepts 1 electron
Real-World Applications
Metallic Applications
- Salt production
- Sodium lamps
- Nuclear reactors
Non-metallic Applications
- Water purification
- PVC production
- Disinfectants
Property Quiz
Which element has higher electrical conductivity?