Surface Energy & Surface Tension
Interactive simulation of molecular interactions in liquids, demonstrating the concepts of surface energy and surface tension
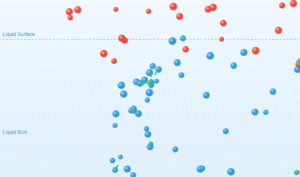
Molecules Inside Liquid (Fig. 9.14a)
Simulation Controls
Molecular Motion
Medium
Interaction Strength
Normal
View Selection
Surface Molecules
50%
Scientific Explanation
Inside the liquid: Molecules are attracted equally in all directions by neighboring molecules, resulting in a net force of zero. This leads to a lower potential energy state.
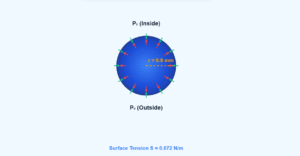
At the surface: Molecules experience unbalanced inward forces due to fewer neighboring molecules above. This creates surface tension and requires extra surface energy.
Surface energy is roughly half the energy needed to completely remove a molecule from the liquid (half the heat of evaporation). For water, this is approximately 40 kJ/mol.