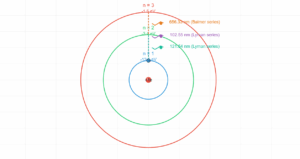
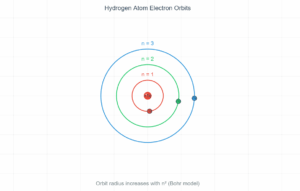
Bohr Model Electron Orbits
Hydrogen Atom Quantum States Simulation
Electron Orbit Visualization
1
Quantum State Calculations
Electron Speed (vₙ)
2.18
×10⁶ m/s
Orbital Radius (rₙ)
5.29
×10⁻¹¹ m
Orbital Period (Tₙ)
1.53
×10⁻¹⁶ s
Energy Level
n=1
Electron Speed: vₙ = e²/(2ε₀hn)
Orbital Radius: rₙ = n²h²ε₀/(πme²)
Orbital Period: Tₙ = 2πrₙ/vₙ
Where: e=1.6×10⁻¹⁹C, ε₀=8.85×10⁻¹²N⁻¹C²m⁻², h=6.62×10⁻³⁴Js, m=9.1×10⁻³¹kg
Orbital Radius: rₙ = n²h²ε₀/(πme²)
Orbital Period: Tₙ = 2πrₙ/vₙ
Where: e=1.6×10⁻¹⁹C, ε₀=8.85×10⁻¹²N⁻¹C²m⁻², h=6.62×10⁻³⁴Js, m=9.1×10⁻³¹kg