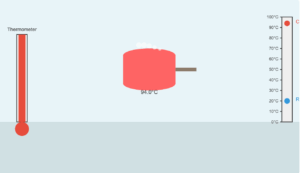
Heat of Fusion Simulation
Example
Question:
When 0.15 kg of ice at \(0^\circ\mathrm{C}\) is mixed with 0.30 kg of water at \(50^\circ\mathrm{C}\) in a container, the resulting temperature is \(6.7^\circ\mathrm{C}\). Calculate the heat of fusion of ice.
(\(s_\mathrm{water} = 4186\,\mathrm{J\,kg^{-1}\,K^{-1}}\))
Solution:
Heat lost by water:
\[
Q_\mathrm{lost} = m_w s_w (\theta_f - \theta_{i,w}) = 0.30 \times 4186 \times (50.0 - 6.7)
= 54,376.14\,\mathrm{J}
\]
Heat required to melt ice:
\[
Q_\mathrm{melt} = m_\mathrm{ice} L_f = 0.15\,L_f
\]
Heat required to raise temperature of melted ice to final temperature:
\[
Q_\mathrm{raise} = m_w s_w (\theta_f - \theta_{i,i}) = 0.15 \times 4186 \times (6.7 - 0)
= 4,206.93\,\mathrm{J}
\]
Heat lost = heat gained:
\[
54,376.14 = 0.15\,L_f + 4,206.93
\]
\[
L_f = \frac{54,376.14 - 4,206.93}{0.15} = 333,795\,\mathrm{J\,kg^{-1}} \approx 3.34 \times 10^5\,\mathrm{J\,kg^{-1}}
\]
Physics Explanation
This simulation demonstrates the heat transfer when ice at 0°C is mixed with water at a higher temperature. The heat lost by the water is equal to the heat gained by the ice to melt and then warm up.
Heat gained by ice = mice × Lf + mice × swater × (θfinal - 0°C)
Where:
- m = mass
- swater = specific heat capacity of water (4186 J kg-1 K-1)
- Lf = heat of fusion of ice
- θ = temperature