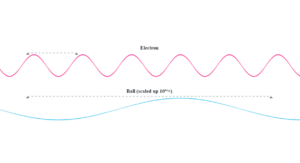
Photoelectric Effect Simulation
This simulation demonstrates the photoelectric effect from Example 11.2. Adjust the material work function and incident light wavelength to see how it affects electron emission. The threshold frequency is calculated as ν₀ = φ₀/h, and the stopping potential relates to the incident light energy through eV₀ = hν - φ₀.
Material Properties
Light Properties
Example
Question:
The work function of caesium is 2.14 eV. Find (a) the threshold frequency for caesium, and (b) the wavelength of the incident light if the photocurrent is brought to zero by a stopping potential of 0.60 V.
Solution:
(a) The threshold frequency is:
\[
\nu_0 = \frac{\phi_0}{h} = \frac{2.14~\text{eV}}{6.63 \times 10^{-34}~\text{Js}}
\]
Convert eV to J:
\[
2.14~\text{eV} = 2.14 \times 1.6 \times 10^{-19}~\text{J}
\]
\[
\nu_0 = \frac{2.14 \times 1.6 \times 10^{-19}}{6.63 \times 10^{-34}} = 5.16 \times 10^{14}~\text{Hz}
\]
(b) When photocurrent goes to zero (stopping potential \(V_0 = 0.60~\text{V}\)), the photoelectric equation gives:
\[
eV_0 = h\nu - \phi_0
\]
Using \(\nu = \frac{c}{\lambda}\):
\[
eV_0 = \frac{hc}{\lambda} - \phi_0
\]
\[
\lambda = \frac{hc}{eV_0 + \phi_0}
\]
Substitute values:
\[
hc = (6.63 \times 10^{-34}~\text{Js})(3 \times 10^8~\text{m/s}) = 19.89 \times 10^{-26}~\text{J m}
\]
Total energy:
\[
(0.60 + 2.14)~\text{eV} = 2.74~\text{eV} = 2.74 \times 1.6 \times 10^{-19}~\text{J}
\]
\[
\lambda = \frac{19.89 \times 10^{-26}}{2.74 \times 1.6 \times 10^{-19}}~\text{m} \approx 454~\text{nm}
\]