Rutherford's Atomic Model Simulation
Example
Question:
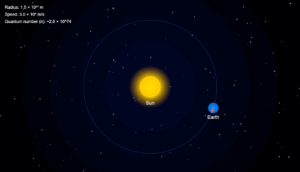
In the Rutherford’s nuclear model of the atom, the nucleus (radius about \( 10^{-15} \) m) is analogous to the sun about which the electron moves in orbit (radius \( \approx 10^{-10} \) m), like the earth orbits around the sun. If the dimensions of the solar system had the same proportions as those of the atom, would the earth be closer to or farther away from the sun than actually it is? The radius of earth’s orbit is about \( 1.5 \times 10^{11} \) m. The radius of sun is taken as \( 7 \times 10^{8} \) m.
Solution:
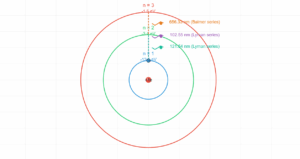
The ratio of the radius of the electron’s orbit to the radius of the nucleus is \( \frac{10^{-10}}{10^{-15}} = 10^{5} \). That is, the radius of electron’s orbit is \( 10^{5} \) times larger than the nucleus.
If the radius of earth’s orbit around the sun were \( 10^{5} \) times larger than the radius of the sun, the earth’s orbit would be \( 10^{5} \times 7 \times 10^{8} = 7 \times 10^{13} \) m.
This is more than 100 times greater than the actual orbital radius of earth (\( 1.5 \times 10^{11} \) m). Thus, earth would be much farther away from the sun.
It implies that an atom contains a much greater fraction of empty space than our solar system does.
Size Comparison:
Nucleus radius: 10-15 m
Electron orbit: 10-10 m
Ratio: 105
Sun radius: 7×108 m
Earth orbit: 1.5×1011 m
Ratio: 214
If scaled to atomic proportions, Earth would be 7×1013 m from the Sun (100× farther than actual).
Physics Explanation:
Rutherford's nuclear model (1911) proposed that:
- Atoms have a tiny, dense, positively charged nucleus
- Electrons orbit the nucleus like planets around the sun
- Most of the atom is empty space
Key findings from the Geiger-Marsden experiment:
- Most alpha particles passed through the gold foil undeflected
- Some were deflected at large angles
- A very few bounced straight back
This led to the conclusion that:
(Electron orbit radius) / (Nucleus radius) ≈ 105
Compared to the solar system ratio of only ~200, showing atoms are much emptier than our solar system.