Gas Molecules RMS Speed Simulation
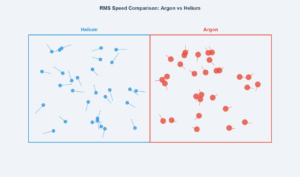
This simulation demonstrates how different gases at the same temperature and pressure have different root mean square speeds due to their molecular masses. The three vessels contain equal numbers of molecules (Avogadro's law), but neon (monatomic) moves fastest while uranium hexafluoride (polyatomic) moves slowest.
Neon (monatomic) RMS speed:
0 m/s
Chlorine (diatomic) RMS speed:
0 m/s
Uranium hexafluoride (polyatomic) RMS speed:
0 m/s
Neon (monatomic)
Chlorine (diatomic)
Uranium hexafluoride (polyatomic)