Electromagnetic Spectrum Photon Energy Simulator
Explore the relationship between wavelength and photon energy across the electromagnetic spectrum. Visualize how energy varies from gamma rays to radio waves using quantum physics principles.
10-12 m (Gamma)
10-2 m (Radio)
Current Measurement
10-6 meters
1.24 × 10-6 eV
(Infrared Region)
Physics Details
▼Photon Energy Calculation
E = hν = hc/λ
Where:
E = Photon energy (J or eV)
h = Planck's constant = 6.62607015 × 10-34 J·s
ν = Frequency (Hz)
c = Speed of light = 299,792,458 m/s
λ = Wavelength (m)
1 eV = 1.602176634 × 10-19 J
Where:
E = Photon energy (J or eV)
h = Planck's constant = 6.62607015 × 10-34 J·s
ν = Frequency (Hz)
c = Speed of light = 299,792,458 m/s
λ = Wavelength (m)
1 eV = 1.602176634 × 10-19 J
Spectrum Characteristics
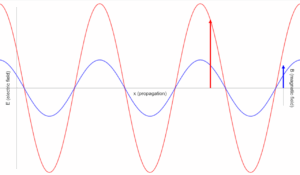
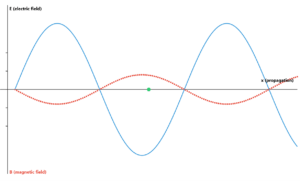
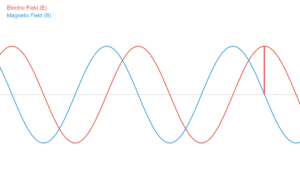
The electromagnetic spectrum is divided into regions based on wavelength and photon energy. Higher energy photons (shorter wavelengths) originate from atomic nuclei (gamma rays) or electron transitions (X-rays, UV). Lower energy photons (longer wavelengths) come from molecular vibrations (infrared) or moving charges (radio waves).
// Energy in electron volts (eV)
function calculateEnergy(wavelength) {
const h = 6.626e-34; // Planck's constant (J·s)
const c = 3e8; // Speed of light (m/s)
const eV = 1.602e-19; // 1 eV in joules
return (h * c) / (wavelength * eV);
}
function calculateEnergy(wavelength) {
const h = 6.626e-34; // Planck's constant (J·s)
const c = 3e8; // Speed of light (m/s)
const eV = 1.602e-19; // 1 eV in joules
return (h * c) / (wavelength * eV);
}