Nuclear Radii Simulation
This simulation explores how atomic nuclei scale with mass number. By comparing the nuclear radii of gold-197 and silver-107, you'll see that larger nuclei aren't proportionally larger—they follow a mathematical relationship called the empirical nuclear radius formula. Understanding nuclear size helps explain nuclear stability, radioactive decay, and fundamental forces that hold atoms together.
Gold-197 Radius
6.23 fm
Silver-107 Radius
5.08 fm
Radius Ratio (Au / Ag)
1.223
How It Works
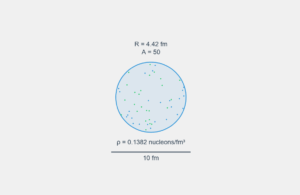
The nuclear radius follows the empirical formula, which predicts that nuclei with more nucleons are only slightly larger, not proportionally larger.
\[ R = R_0 \, A^{1/3} \]
Where:
- R = nuclear radius
- R₀ ≈ 1.2 femtometers (fm) — empirical constant
- A = mass number (total nucleons)
For our isotopes:
- Gold-197: R = 1.2 × 197^(1/3) ≈ 6.23 fm
- Silver-107: R = 1.2 × 107^(1/3) ≈ 5.08 fm
- Ratio: 6.23 / 5.08 ≈ 1.22