Hydrogen Spectrum Simulation
Visualizing quantum transitions and spectral emissions
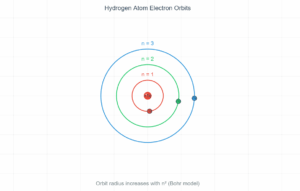
This simulation demonstrates the electron transitions in a hydrogen atom when excited by a 12.5eV electron beam. The electron jumps from the ground state (n=1) to the n=3 energy level, then emits photons as it returns to lower energy states, producing characteristic spectral lines.
Transition Information
Select a transition from the dropdown to view detailed information about the spectral line it produces.
656.33 nm Balmer series
This is the first step transition from n=3 to n=2, emitting visible red light at 656.33 nm (Balmer series).
- Initial Energy (n=3): -1.5 eV
- Final Energy (n=2): -3.4 eV
- Energy Difference: 1.9 eV