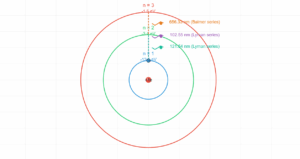
Hydrogen Atom Orbit Radii
Visualizing n=1, n=2, and n=3 orbits
This simulation shows the relative sizes of electron orbits in a hydrogen atom for principal quantum numbers n=1, n=2, and n=3. The radius increases with n², as predicted by Bohr's model.
Orbit Radii Information
n=1 Orbit (Ground State)
5.3 × 10⁻¹¹ m
Innermost electron orbit with smallest radius
n=2 Orbit
2.1 × 10⁻¹⁰ m
4 times larger than n=1 (2² × r₁)
n=3 Orbit
4.77 × 10⁻¹⁰ m
9 times larger than n=1 (3² × r₁)