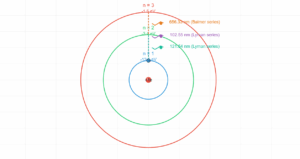
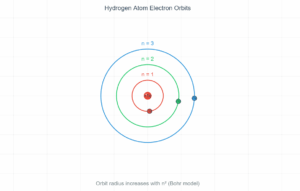
Hydrogen Atom Energy Level Transition
Quantum Physics Simulation | Bohr Model Visualization
Simulation Canvas
Energy Level Controls
1
4
Quantum Transition Data
Energy Difference (ΔE)
12.75
eV
Photon Wavelength (λ)
97.3
nm
Photon Frequency (ν)
3.09
×10¹⁵ Hz
Photon Energy
2.04
×10⁻¹⁸ J
Energy Level Formula: Eₙ = -13.6 eV × (1/n²)
Energy Difference: ΔE = E₂ - E₁ = 13.6 eV × (1/n₁² - 1/n₂²)
Photon Wavelength: λ = hc/ΔE
Photon Frequency: ν = ΔE/h
Energy Difference: ΔE = E₂ - E₁ = 13.6 eV × (1/n₁² - 1/n₂²)
Photon Wavelength: λ = hc/ΔE
Photon Frequency: ν = ΔE/h
Transition Diagram