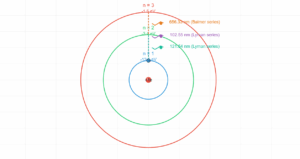
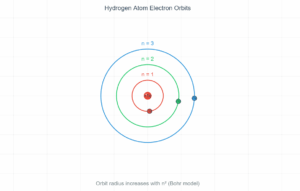
Atomic Energy Level Transition
0.1 eV
2.3 eV
5.0 eV
Transition Information
When an electron transitions from a higher energy level to a lower one, it emits a photon with energy equal to the difference between the levels.
E = hν
Where:
E = Energy difference (2.3 eV = 3.68 × 10⁻¹⁹ J)
h = Planck's constant (6.626 × 10⁻³⁴ Js)
ν = Frequency of emitted radiation
Calculated frequency: 5.55 × 10¹⁴ Hz