Photoelectric Threshold Simulation
Metal Properties
Value: 4.2 eV
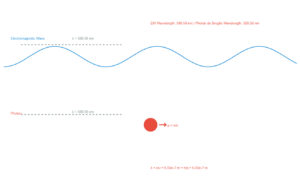
Incident Radiation
Value: 330 nm
Photoelectric Effect Theory
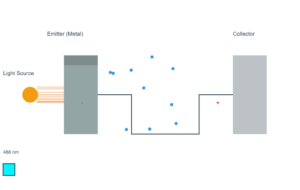
The photoelectric effect occurs when light of sufficient energy (above the work function) shines on a metal surface, causing electrons to be emitted.
Key Equations
Photon Energy: E = hν = hc/λ
Where:
- h = Planck's constant (6.626 × 10⁻³⁴ J·s)
- c = speed of light (3 × 10⁸ m/s)
- λ = wavelength of incident radiation
Emission Condition: hν ≥ φ
Where φ is the work function of the metal.
Current Simulation Results
Work Function (φ): 4.2 eV
Incident Photon Energy: 3.76 eV
Emission Status: NO EMISSION (3.76 eV < 4.2 eV)
Kinetic Energy of Emitted Electrons: 0.00 eV