Nuclear Decay Simulation
Example
Question:
(a) Are the equations of nuclear reactions (such as those given in Section 13.7) ‘balanced’ in the sense a chemical equation (e.g., \(2\text{H}_2 + \text{O}_2 \to 2\text{H}_2\text{O}\)) is? If not, in what sense are they balanced on both sides?
(b) If both the number of protons and the number of neutrons are conserved in each nuclear reaction, in what way is mass converted into energy (or vice-versa) in a nuclear reaction?
(c) A general impression exists that mass-energy interconversion takes place only in nuclear reaction and never in chemical reaction. This is strictly speaking, incorrect. Explain.
Solution:
(a) A chemical equation is balanced such that the number of atoms of each element is the same on both sides. In a nuclear reaction, elements may be transmuted, so the atom count is not necessarily conserved. However, the number of protons and neutrons are both (separately) conserved in a nuclear reaction (apart from rare high-energy exceptions, where total charge and baryon number are strictly conserved). Therefore, nuclear reactions are "balanced" in terms of the number of protons and neutrons.
(b) The binding energy of a nucleus (mass defect) contributes negatively to the total mass. Even though the total number of protons and neutrons are conserved, the total rest mass on each side can differ due to different binding energies. The difference appears as energy released or absorbed, thus exemplifying mass-energy interconversion (\(E=mc^2\)).
(c) Chemical reactions also involve mass-energy interconversion, in principle. Chemical binding energy gives a (very small) negative mass contribution. The energy released or absorbed in a chemical reaction comes from the difference in chemical binding energies. Thus, the difference in total mass of atoms or molecules on each side of the chemical reaction gets converted into energy or vice versa, but the effect is a million times smaller than in nuclear reactions, which is why it is usually ignored in chemistry.
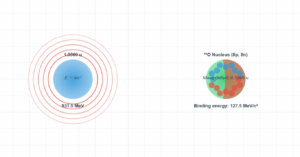
Nuclear Decay Processes
This 3D visualization demonstrates nuclear decay processes. Alpha decay occurs when an unstable nucleus emits an alpha particle (helium nucleus). Proton emission is a rare decay mode where a proton is ejected from the nucleus. The simulation shows that alpha decay of Uranium-238 releases 4.25 MeV of energy, while proton emission is not energetically possible (Q = -7.68 MeV) for this isotope.