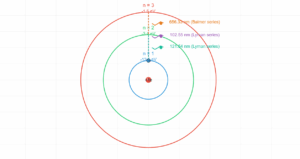
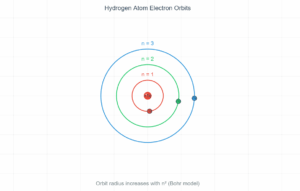
Hydrogen Atom Energy Levels
Energy Level Diagram
Rydberg Formula for Hydrogen:
\[ \frac{1}{\lambda} = R_H \left( \frac{1}{n_f^2} - \frac{1}{n_i^2} \right) \]
Energy Levels:
\[ E_n = -\frac{13.6 \text{ eV}}{n^2} \]
Where:
- λ is the wavelength of emitted light
- nf is the final energy level
- ni is the initial energy level (ni > nf)
- RH is the Rydberg constant (1.097 × 107 m-1)