Surface Tension and Contact Angle Simulation
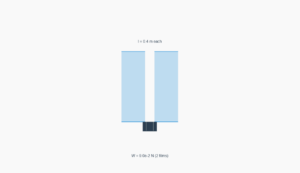
Water on glass: Acute contact angle (wetting)
Question 2a
Explain why the angle of contact of mercury with glass is obtuse, while that of water with glass is acute.
The contact angle (θ) is determined by the balance between three interfacial tensions:
γSA = γSL + γLAcosθ
Where:
- γSA = solid-air interfacial tension
- γSL = solid-liquid interfacial tension
- γLA = liquid-air interfacial tension (surface tension)
For water-glass: γSA > γSL, so cosθ is positive → θ is acute (0-30°)
For mercury-glass: γSA < γSL, so cosθ is negative → θ is obtuse (~140°)
Question 2b
Why does water wet glass while mercury does not?
Wetting occurs when the liquid spreads on the solid surface, which happens when:
γSA > γSL + γLA
Water wets glass because water molecules are strongly attracted to the polar glass surface (adhesive forces > cohesive forces).
Mercury doesn't wet glass because mercury atoms have much stronger cohesive forces (between mercury atoms) than adhesive forces (to glass).
Question 2c
Why is surface tension independent of surface area?
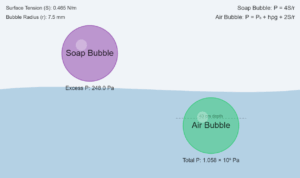
Surface tension (γ) is defined as the force per unit length (N/m) acting at the surface, or equivalently the energy per unit area (J/m²).
It depends only on the nature of the liquid and temperature, not on the area because:
- Surface tension arises from intermolecular forces at the surface
- These forces are short-range and depend only on local molecular environment
- Increasing area simply creates more identical surface with the same properties
Question 2d
Why does water with detergent have small contact angles?
Detergents reduce surface tension (γLA) and also reduce γSL by adsorbing at the interfaces:
- Detergent molecules are amphiphilic (have both hydrophilic and hydrophobic parts)
- They accumulate at interfaces, reducing interfacial tensions
- From Young's equation: γSA = γSL + γLAcosθ
- Reduction in γLA and γSL makes cosθ approach 1 → θ approaches 0°
Question 2e
Why is a free liquid drop always spherical?
In the absence of external forces, surface tension minimizes the surface area for a given volume:
- Surface tension acts like an elastic skin trying to minimize surface area
- For a given volume, the shape with smallest surface area is a sphere
- This is why small droplets (where surface forces dominate over gravity) are spherical
- Larger drops may flatten due to gravity overcoming surface tension