Free Expansion Gas Simulation
Joule expansion of gas between connected cylinders
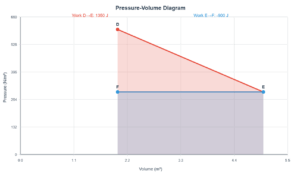
Final Pressure
1.0 atm
Internal Energy
0 ΔU
Temperature
0 ΔT
Key Points:
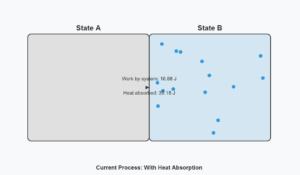
- Free expansion is an irreversible process where gas expands into a vacuum
- No work is done (W = 0) and no heat is transferred (Q = 0)
- Internal energy remains constant (ΔU = 0)
- Temperature remains unchanged for an ideal gas
- Pressure halves when volume doubles (P₂ = P₁V₁/V₂)