Thermodynamics Process Simulation
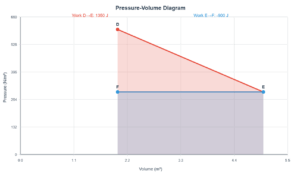
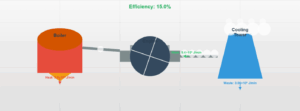
This simulation demonstrates the transition of a gas from state A to state B through two different processes: an adiabatic process (no heat transfer) and a process with heat absorption. The first law of thermodynamics (ΔU = Q - W) governs these transitions.
22.3
Adiabatic Process
Work done on system: 22.3 J
Heat transferred (ΔQ): 0 J
Internal energy change (ΔU): 22.3 J
Process with Heat
Heat absorbed (ΔQ): 39.18 J
Work done by system: 16.88 J
Internal energy change (ΔU): 22.3 J
First Law of Thermodynamics: ΔU = Q - W
Where: ΔU = Change in internal energy, Q = Heat added to system, W = Work done by system