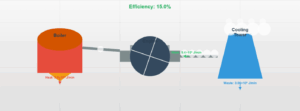
Nitrogen Gas Heating Simulation
Interactive visualization of heat transfer in diatomic nitrogen (N₂) at constant pressure
Particle Speed
0 m/s
Kinetic Energy
0 J
Number of Moles
0.714 mol
Particle Count
60
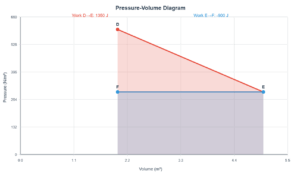
Thermodynamics Calculations
This simulation demonstrates the heating of nitrogen gas (N₂) at constant pressure using the following thermodynamic principles:
Number of moles: n = m/M = mass / molar mass
Molar heat capacity at constant pressure: Cₚ = (7/2)R = 29.05 J·mol⁻¹·K⁻¹
Heat required: ΔQ = n·Cₚ·ΔT
Heat required to raise temperature by 0°C: 0 J
Molar heat capacity (Cₚ): 29.05 J·mol⁻¹·K⁻¹