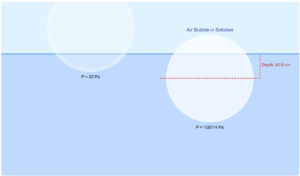
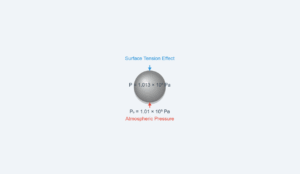
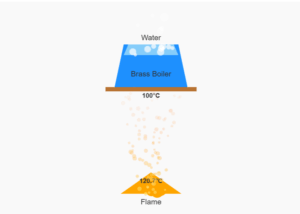
Specific Heat Experiment Simulation
100g
200g
300g
100°C
150°C
200°C
10°C
27°C
40°C
Results:
Final Temperature: 40°C
Calculated Specific Heat: 0.43 J/g°C
Heat lost by metal = Heat gained by water + calorimeter
m₁c₁ΔT₁ = (m₂ + m₃)c₂ΔT₂