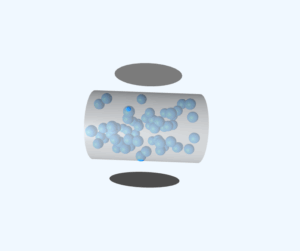
Mean Free Path Simulation
This simulation visualizes the concept of mean free path - the average distance a molecule travels between collisions. The example shows water vapor molecules at 373K with a mean free path of approximately 400nm (100 times the molecular diameter).
Gas Properties
Simulation Settings
Example
Question:
Estimate the mean free path for a water molecule in water vapour at 373 K. Use information from Exercises 12.1 and Eq. (12.41) above.
Solution:
For water vapour, the effective molecular diameter \(d\) is the same as air. The number density is inversely proportional to temperature:
\[
n = 2.7 \times 10^{25} \times \frac{273}{373} = 2 \times 10^{25}\,\mathrm{m}^{-3}
\]
Hence, mean free path
\[
l = 4 \times 10^{-7}\,\mathrm{m}
\]
Note: The mean free path is 100 times the interatomic distance (\(\sim 40\,\text{\AA} = 4 \times 10^{-9}\,\mathrm{m}\)) previously calculated. This large mean free path explains typical gaseous behaviour—gases cannot be confined without a container.
Simulation Information
Mean Free Path: 4.00 ×10⁻⁷ m (400nm)
Collisions per second: 0
Average speed: 0 m/s