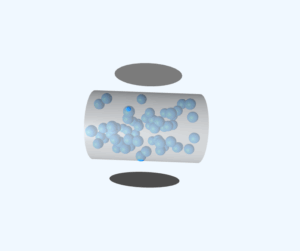
Gas Mixture Simulation
Interactive visualization of neon (Ne) and oxygen (O₂) gas molecules demonstrating partial pressure relationships and molecular behavior.
Animation Controls
Gas Mixture
Example
Question:
A vessel contains two non-reactive gases: neon (monatomic) and oxygen (diatomic). The ratio of their partial pressures is 3:2. Estimate the ratio of (i) number of molecules and (ii) mass density of neon and oxygen in the vessel. Atomic mass of Ne = 20.2 u, molecular mass of O\(_2\) = 32.0 u.
Solution:
The partial pressure ratio is \( \frac{P_1}{P_2} = \frac{3}{2} \).
(i) By the ideal gas law,
\[
\frac{P_1}{P_2} = \frac{\mu_1}{\mu_2}
\]
where \( \mu_1 = \frac{N_1}{N_A} \) and \( \mu_2 = \frac{N_2}{N_A} \) are mole numbers. Thus,
\[
\frac{N_1}{N_2} = \frac{\mu_1}{\mu_2} = \frac{3}{2}
\]
(ii) For mass density,
\[
\frac{\rho_1}{\rho_2} = \frac{m_1/V}{m_2/V} = \frac{m_1}{m_2} = \frac{\mu_1}{\mu_2} \times \frac{M_1}{M_2}
\]
where \( M_1 = 20.2~\text{u} \), \( M_2 = 32.0~\text{u} \),
\[
\frac{\rho_1}{\rho_2} = \frac{3}{2} \times \frac{20.2}{32.0} = 0.947
\]
Real-time Calculations
About This Simulation
This simulation demonstrates key principles of gas mixture behavior:
- Partial Pressure: Each gas contributes to total pressure proportional to its molecular count
- Molecular Motion: Particles move randomly following kinetic theory principles
- Density Relationships: Gas density depends on both molecular count and molecular mass
- Temperature Effects: Higher temperature increases average particle velocities