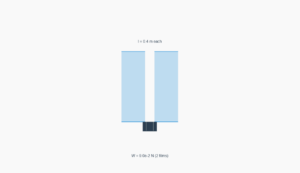
Capillary Action Simulation
Demonstrates water rising in a capillary tube due to surface tension. Adjust parameters to see how they affect the meniscus height.
Example
Question:
The lower end of a capillary tube of diameter 2.00 mm is dipped 8.00 cm below the surface of water in a beaker. What is the pressure required in the tube in order to blow a hemispherical bubble at its end in water? The surface tension of water at temperature of the experiment is \(7.30 \times 10^{-2}\,\mathrm{N\,m^{-1}}\), 1 atmospheric pressure = \(1.01 \times 10^5\,\mathrm{Pa}\), density of water = \(1000\,\mathrm{kg\,m^{-3}}\), \(g = 9.80\,\mathrm{m\,s^{-2}}\). Also calculate the excess pressure.
Solution:
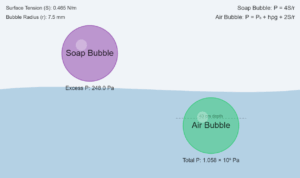
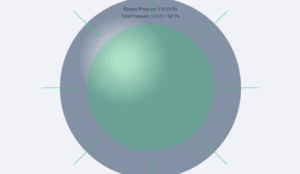
The excess pressure in a bubble of gas in a liquid is given by
\[
\Delta P = \frac{2S}{r}
\]
where \(S\) is the surface tension.
Radius of bubble: \(r = 1.00\,\mathrm{mm} = 1.00 \times 10^{-3}\,\mathrm{m}\).
Pressure outside the bubble (\(P_o\)):
\[
P_o = (1.01 \times 10^5\,\mathrm{Pa}) + (0.08\,\mathrm{m} \times 1000\,\mathrm{kg\,m^{-3}} \times 9.80\,\mathrm{m\,s^{-2}})
= 1.01784 \times 10^5\,\mathrm{Pa}
\]
Pressure inside the bubble (\(P_i\)):
\[
P_i = P_o + \frac{2S}{r}
\]
\[
= 1.01784 \times 10^5\,\mathrm{Pa} + \frac{2 \times 7.3 \times 10^{-2}\,\mathrm{N\,m^{-1}}}{1.0 \times 10^{-3}\,\mathrm{m}}
= 1.01784 \times 10^5\,\mathrm{Pa} + 146\,\mathrm{Pa}
= 1.02 \times 10^5\,\mathrm{Pa}
\]
The excess pressure in the bubble is \(146\,\mathrm{Pa}\).